-
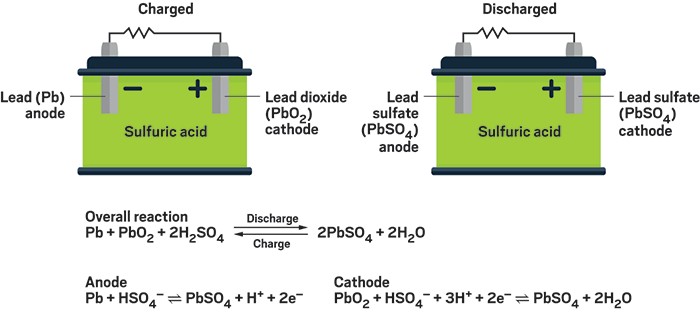
 Solved The standard half-cell reactions lead acid battery | Chegg.com
Solved The standard half-cell reactions lead acid battery | Chegg.com
ID: 6hziM7dBe4
From: chegg.com
-
 What Lead-Acid Battery? - Construction & Charging with Videos
What Lead-Acid Battery? - Construction & Charging with Videos
ID: f7ybiAqMoW
From: byjus.com
-
 How does a lead-acid battery work by stating reactions that at and cathode respectively during charging discharging? What is the valence state for lead involved in the reaction? -
How does a lead-acid battery work by stating reactions that at and cathode respectively during charging discharging? What is the valence state for lead involved in the reaction? -
ID: tKXZ9jpFhb
From: quora.com
-
 electrochemistry - Redox and anode/cathode in lead acid Exchange
electrochemistry - Redox and anode/cathode in lead acid Exchange
ID: CoYLjMt4yV
From: chemistry.stackexchange.com
-
 Acid Battery
Acid Battery
ID: 4qtHRU7cjY
From: electricalschool.org
-
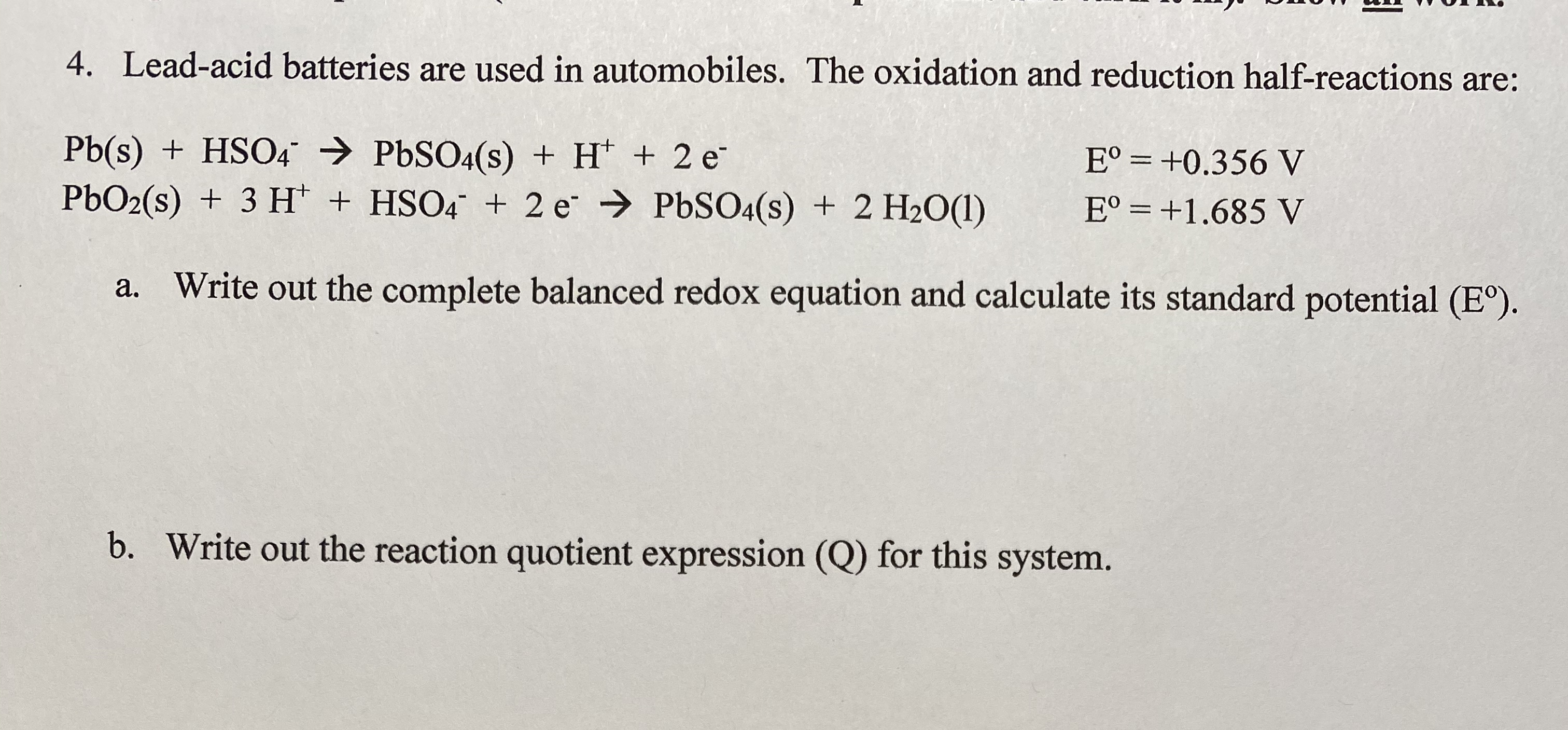 Answered: 4. batteries are used | bartleby
Answered: 4. batteries are used | bartleby
ID: DPNVtpzAIt
From: bartleby.com
-
 Lead storage battery | Redox reactions and electrochemistry Chemistry | Khan Academy - YouTube
Lead storage battery | Redox reactions and electrochemistry Chemistry | Khan Academy - YouTube
ID: Lvls2AQchg
From: youtube.com
-
 Working of Lead Acid Battery | Lead Acid Storage Battery | Electrical4U
Working of Lead Acid Battery | Lead Acid Storage Battery | Electrical4U
ID: 3R435A4hTW
From: electrical4u.com
-
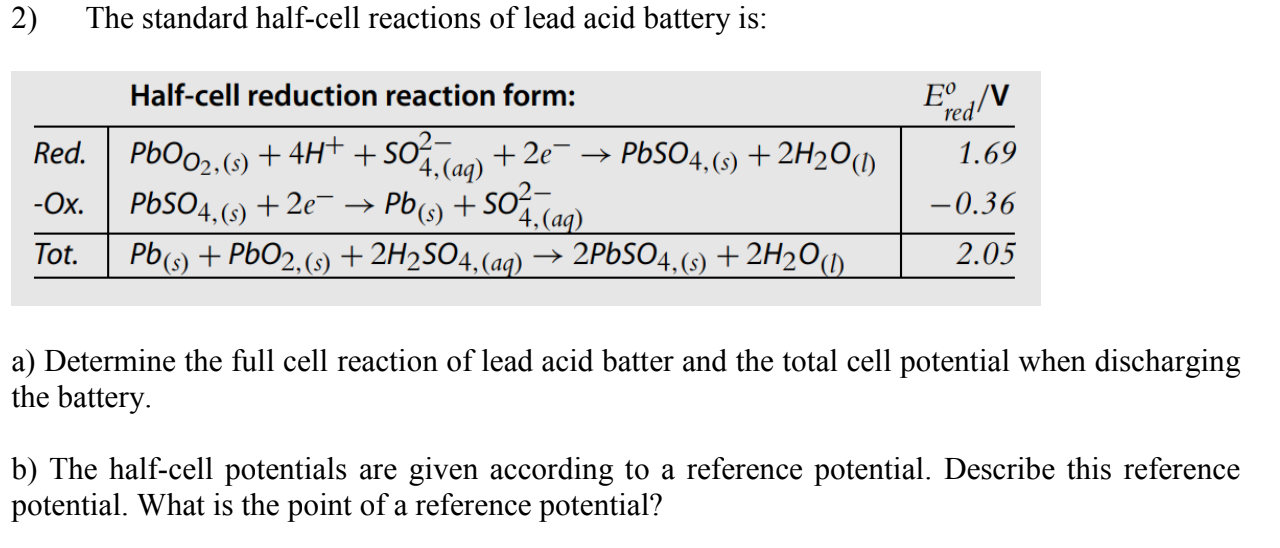 Solved The standard half-cell reactions lead acid battery | Chegg.com
Solved The standard half-cell reactions lead acid battery | Chegg.com
ID: 2ukrZAhrUr
From: chegg.com
-
 The reactions occur in a acid battery are: PbSO(3)(s)
The reactions occur in a acid battery are: PbSO(3)(s)
ID: Lp6ixasEZe
From: doubtnut.com
-
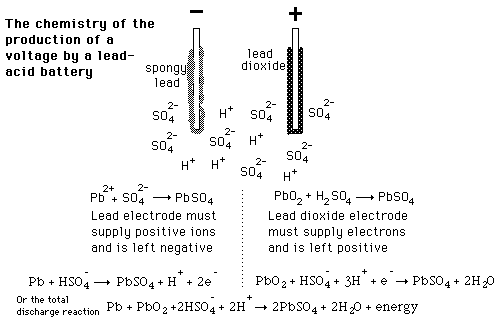 Lead-Acid
Lead-Acid
ID: BF3tkZEWof
From: hyperphysics.phy-astr.gsu.edu
-
Solved Two half-cell reaction for a lead acid is | Chegg.com
ID: q0GstY00bb
From: chegg.com
-
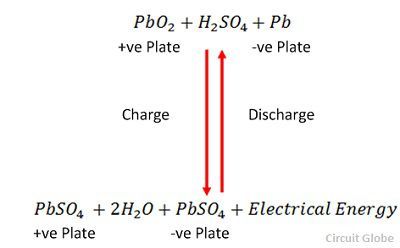 What Lead Acid Battery? Construction, Discharging & Recharging - Circuit Globe
What Lead Acid Battery? Construction, Discharging & Recharging - Circuit Globe
ID: uYuUaPPlIT
From: circuitglobe.com
-
ID: dCdbxUbif0
From: cen.acs.org
-
in General Chemistry for Austine #348476
ID: 3xM10A1BXH
From: assignmentexpert.com
-
 Battery Charging and
Battery Charging and
ID: hIUNtMkNTJ
From: siranah.de
-
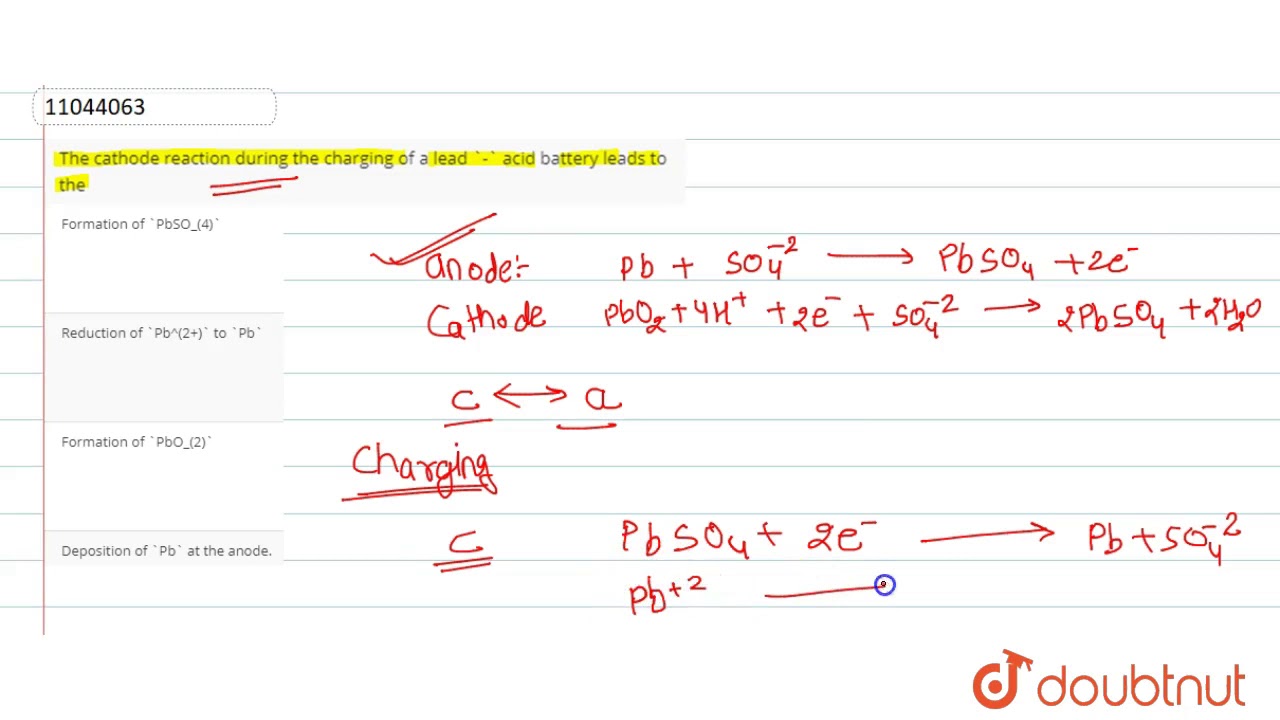 cathode during the charging of a lead `-` acid battery leads to the - YouTube
cathode during the charging of a lead `-` acid battery leads to the - YouTube
ID: SqhfH9GhYc
From: youtube.com
-
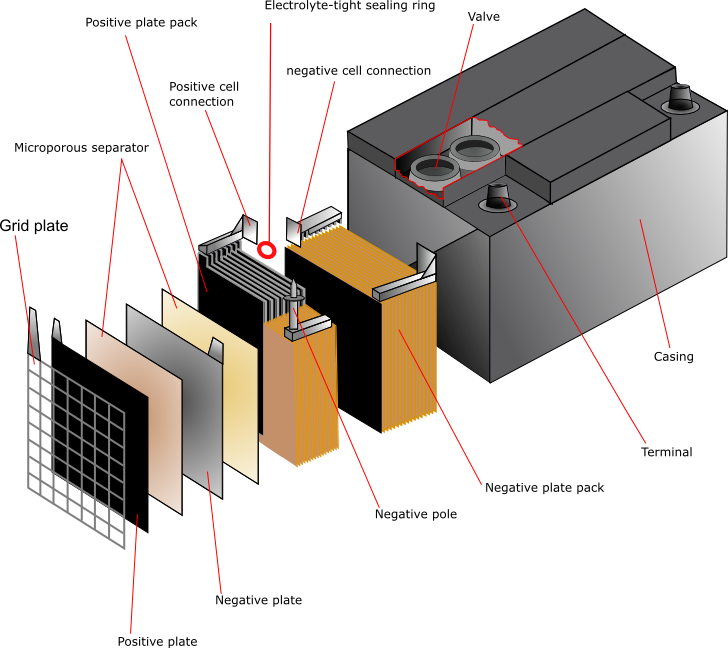 batteries
batteries
ID: oaTMdRwexX
From: doitpoms.ac.uk
-
 Battery Charging and
Battery Charging and
ID: 1yBJAroCeI
From: siranah.de
-
 The half reactions that occur in a lead battery are: `PbSO_(3)(s)+2e^(-)toPb(s)+SO_(4) - YouTube
The half reactions that occur in a lead battery are: `PbSO_(3)(s)+2e^(-)toPb(s)+SO_(4) - YouTube
ID: l7GbyvJrkG
From: youtube.com
-
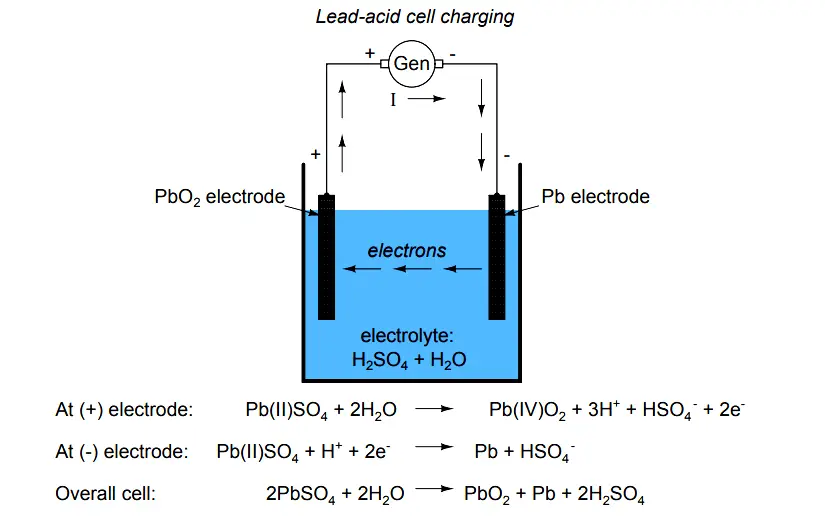 Electron Activity in Chemical Reactions - Lead acid Cell
Electron Activity in Chemical Reactions - Lead acid Cell
ID: pvTzd8gYrs
From: instrumentationtools.com
-
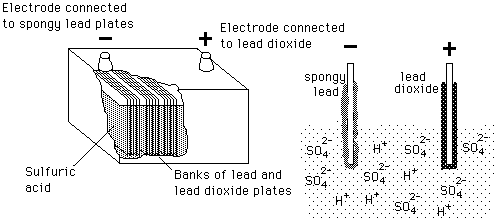 Lead-Acid
Lead-Acid
ID: QbQTAzjv8F
From: hyperphysics.phy-astr.gsu.edu
-
 Answered: 4. batteries are used | bartleby
Answered: 4. batteries are used | bartleby
ID: 8X1vWOfwyZ
From: bartleby.com
-
 1 UNIT / Oxidation Reactions “Redox” and Electrochemistry. ppt download
1 UNIT / Oxidation Reactions “Redox” and Electrochemistry. ppt download
ID: P3d3HozLrI
From: slideplayer.com
-
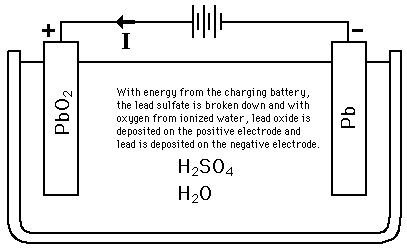 Lead-Acid
Lead-Acid
ID: WpUMHc407Q
From: hyperphysics.phy-astr.gsu.edu
-
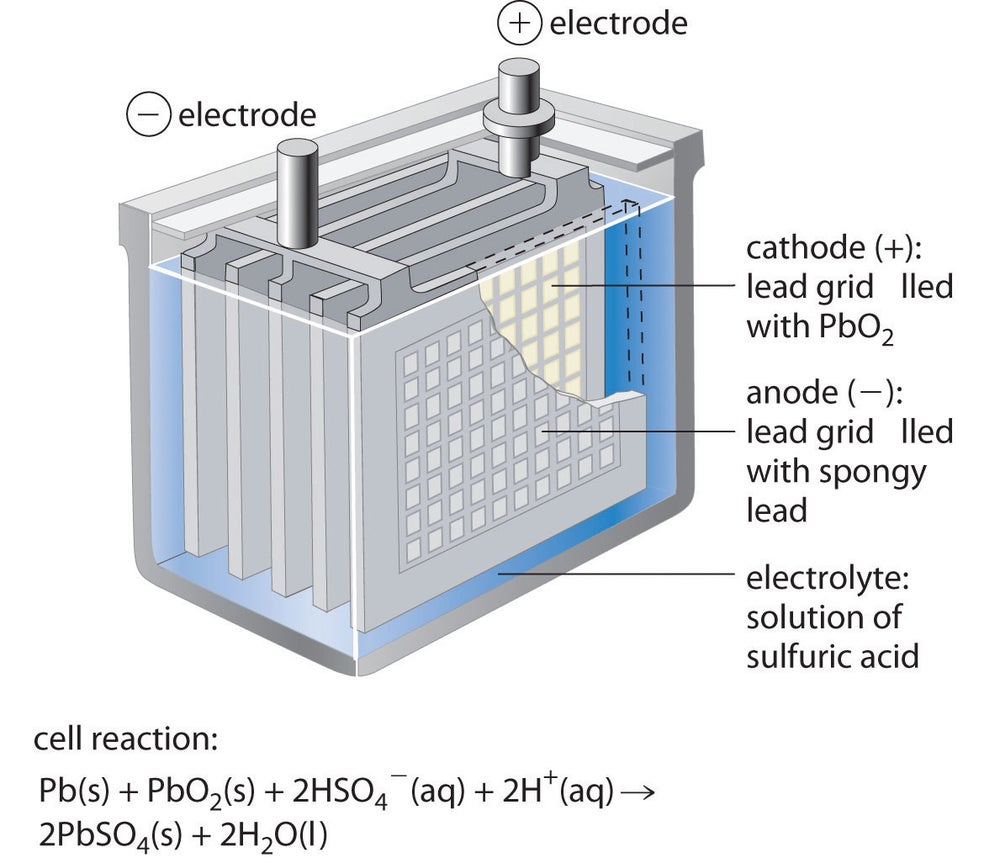 Should You Choose A Acid Battery Solar Storage?
Should You Choose A Acid Battery Solar Storage?
ID: FNUYGryTcs
From: solarreviews.com